 |
Allogeneic HSCT recipients receiving immunosuppressive therapy for the prevention or treatment of GVHD, nontransplant patients with advanced or refractory underlying diseases extensively treated with multiple courses of chemotherapy, and any patient with anticipated prolonged neutropenia are candidates for antifungal prophylaxis. In contrast, autologous HSCT recipients receiving hematopoietic growth factors and solid-tumor patients on chemotherapeutic regimens that cause only short-term neutropenia are at low risk for IFI and usually do not require routine antifungal prophylaxis.
Agents initially used to reduce fungal colonization included oral nystatin, ketoconazole, and amphotericin B (AmB). Prophylaxis with these agents has generally produced inconsistent results due either to lack of efficacy or to poor compliance64 and is not recommended in the current CDC guidelines.57
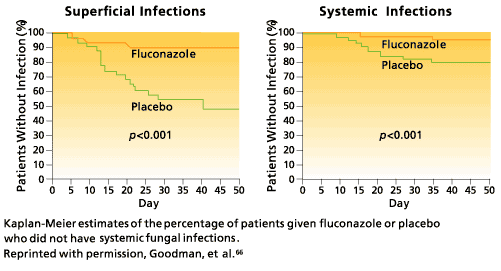
Fluconazole has been the agent most commonly used for prophylaxis of fungal infections. In 2 randomized, double-blind, placebo-controlled trials that enrolled HSCT patients, prophylactic fluconazole significantly reduced the incidence of both systemic and superficial fungal infections (Fig 9),65,66 improved overall survival,65 and was associated with fewer deaths related to fungal infection.66 A dose of 400 mg daily (by mouth or intravenously) is recommended from the day of HSCT until engraftment for prophylaxis against fluconazole-susceptible Candida species.57 Risk for IFI extends beyond the initial period of posttransplant neutropenia, and continuation of fluconazole to day 75 after transplant was shown to improve survival in one study of high-risk allogeneic patients.67

Similar randomized, placebo-controlled trials in patients with acute leukemia have demonstrated the efficacy of prophylactic fluconazole for reducing fungal colonization and preventing superficial infections but were not powered sufficiently to show a decrease in systemic fungal infections.68-70 Other studies have also shown fluconazole to be more effective and better tolerated than nystatin, clotrimazole, and oral AmB for prophylaxis.71-73
Itraconazole, available as an oral suspension, an intravenous formulation, and the older capsule formulation, has also been used for antifungal prophylaxis. In initial trials, the capsules were found to be erratically absorbed by many patients and associated with ineffective serum itraconazole concentrations.74-76 The oral suspension results in adequate serum itraconazole concentrations in neutropenic patients when administered on an empty stomach.78,78 In 2 randomized, double-blind, placebo-controlled trials involving neutropenic patients with hematologic malignancies, itraconazole oral solution effectively prevented superficial and systemic fungal infections.79,80 Another study found that itraconazole oral solution and an oral solution of fluconazole provided similar effectiveness for antifungal prophylaxis.81 None of the trials included a sufficient number of patients at high risk for aspergillosis to demonstrate a decrease in Aspergillus infections.
Amphotericin B administered either as an aerosol or by the intranasal route has also been used prophylactically in neutropenic patients receiving chemotherapy or undergoing HSCT. In 2 trials using historical comparisons, an intranasal AmB spray appeared to be associated with a decreased incidence of Aspergillus infections.82,83 However, in a more recent multicenter prospective, randomized trial, no benefit was found for prophylactic aerosolized AmB.84 The overall incidence of invasive aspergillosis was low in this trial (4% in patients who received prophylactic aerosolized AmB, and 7% in patients who received no inhalation prophylaxis). Consequently, neither intranasal AmB nor aerosolized AmB can be currently recommended for prophylaxis.
Several studies using historical comparisons claimed that prophylaxis with low doses of intravenous AmB (0.1 to 0.25 mg/kg/day) reduced IFI after allogeneic HSCT.19,85 However, in a randomized trial, low doses of prophylactic intravenous AmB (0.1 mg/kg/day) did not demonstrate benefits after autologous HSCT.86 In 2 randomized, double-blind, placebo-controlled trials, prophylactic liposomal AmB decreased fungal colonization without a significant reduction of systemic IFI or mortality.87,88 When low-dose prophylactic intravenous AmB was compared with fluconazole for prevention of fungal infections, AmB was no more effective and had significantly greater toxicity.89,90 The higher cost of lipid formulations of AmB and inconsistencies in clinical trials limit their use as prophylactic antifungal agents. Currently, the only clear indication for prophylactic intravenous AmB is in patients with prior Aspergillus infection who are receiving additional chemotherapy or undergoing HSCT.91
Course Number: V035D
This CME Expires on July 1, 2005; no tests will be accepted after this date.
This course is accredited by
The University of Pittsburgh School of Medicine, Center for Continuing Education and The International Immunocompromised Host Society
|
 |

