Dr. Ostrosky began by presenting a historical perspective of antifungal polyene development,
and highlighted the fact that even though amphotericin B deoxycholate remains
the gold standard for antifungal therapy, it was licensed based upon open-label
studies, and probably would not be licensed by the FDA today. Similarly, the
LFABs were licensed for treatment of IFI based upon open-label trials. In the
past, this has prevented them from being used as comparators in the primary
treatment of IFI in clinical trials by the FDA.
Recently, an article published in the journal
Clinical Infectious Diseases outlined the
FDA challenge.
1 While
it is not an official FDA position, it says that an LFAB can be used as a
comparator if a prospective trial sets out to prove one of two criteria: 1) the
new drug is superior to an LFAB, or 2) the new drug has equivalent efficacy but
is safer than the drug being tested. For example, liposomal amphotericin B was
recently used as a comparator in an empirical antifungal therapy trial with
voriconazole.
2 This
alternative method of performing clinical trials also has the potential of
providing clinicians with more data on the LFABs in order to allow them to make
better decisions about their use.
In a review of the literature, Dr. Ostrosky demonstrated that the LFABs had consistently higher MICs and MFCs
(minimum fungicidal concentrations) in vitro than conventional amphotericin B
(CAB). Subsequent animal models showed that higher doses of the LFABs were
needed compared to CAB, but that these higher doses were well tolerated.
Considering the incidence of amphotericin B-induced nephrotoxicity, clinical
trials have shown that the incidence of nephrotoxicity associated with CAB is
about 30% and results in expensive outcomes.
3,4 In head-to-head comparisons of the LFABs and CAB,
there is consistent data demonstrating a lower incidence of nephrotoxicity with
the LFABs.
5,6
Dr. Ostrosky also reported on 11 open-label trials and 6 head-to-head comparisons demonstrating efficacy of the
LFABs. In the figure, an imaginary line is created at 50%, which is the average
response rate for CAB between all the fungal organisms. All trials except one
had response rates greater than 50%.
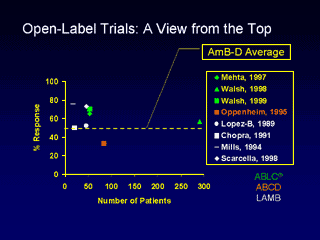
ABLC® is a registered trademark of The Liposome Company, Inc.
A meta-analysis conducted by Dr. Ostrosky and his colleagues reported response rates for Aspergillus of 34% in 32 patients treated with Amphotec (ABCD), 46% in 163 patients treated with Abelcet (ABLC), and 61% in 84 patients treated with AmBisome (LAMB). Herbrecht and colleagues reported a response rate of 32% in patients treated with CAB.
7 In
Candida infections, the response rates were 58% in 33 patients treated with Amphotec, 75% in 123 patients treated with Abelcet, and 80% in 70 patients treated with AmBisome. Rex and colleagues reported a response rate of 79% in patients treated with CAB.
8
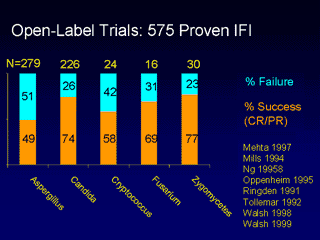
To assess complete or partial response rates in proven cases of IFI, Dr. Ostrosky extracted 575 cases from the literature. From these
cases, the response rate was 49% for Aspergillus, and 74% for Candida.
In general, Dr. Ostrosky feels that the FDA challenge has been met. The LFABs have shown a clear safety advantage over CAB in both
pre-clinical and clinical data. In terms of efficacy, there is also a clear
advantage for the LFABs against
Aspergillus, and equal efficacy against
Candida
and
Cryptococcus. As well, liposomal amphotericin B has shown a clear advantage
over CAB against histoplasmosis. In Dr. Ostrosky’s opinion, these facts should make physicians and the FDA reconsider the continued use of CAB as a therapeutic
gold standard or as the standard comparator in antifungal clinical trials.
References:
1. Powers
J. Counterpoint: Alternative trial designs for antifungal drugs—Time to
talk. Clin Infect Dis 2001;33:107–9.
2. Walsh
TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal
amphotericin B for empirical antifungal therapy in patients with neutropenia
and persistent fever. N Engl J Med 2002;346(4):225–34.
3. Bates
DW, et al. Mortality and costs of acute renal failure associated with
amphotericin B therapy. Clin Infect Dis 2001;32:686–93.
4.
Wingard JR, et al. Clinical significance of nephrotoxicity in patients
treated with amphotericin B for suspected or proven aspergillosis. Clin Infect
Dis 1999;29:1402–7.
5.
Wingard JR, et al. A randomized, double-blind comparative trial
evaluating the safety of liposomal amphotericin B versus amphotericin B lipid
complex in the empirical treatment of febrile neutropenia. Clin Infect Dis
2003;31:1155–63.
6. Walsh
TJ, et al. Liposomal amphotericin B for empirical therapy in patients with
persistent fever and neutropenia. N Engl J Med 1999;340:764–71.
7. Herbrecht
R, Denning DW, Patterson TF, et al. Open, randomised comparison of voriconazole
(VRC) and amphotericin B (AmB) followed by other licensed antifungal therapy
(OLAT) for primary therapy of invasive aspergillosis (IA). Proceedings of the
41st ICAAC 2001;[abstract J-680].
8. Rex J,
et al. A randomized trial comparing fluconazole with amphotericin B for the
treatment of candidemia in patients without neutropenia. Candidemia Study Group
and the National Institute. N Engl J Med 1994;331(20):1325–30.
Conference Organizer : Imedex® education is the best medicine |